| | Updated: Balneo Mary Bath |  |
|
|
|
| Author | Message |
|---|
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Sun Jan 18, 2009 8:24 am Sun Jan 18, 2009 8:24 am | |
| On the subject of dew salt: La rosée
That's french, from a book by Athorene. It shows the salt crystallized, and performs a distillation measuring conductimetry in the distillate. There are 2 inflexion points in the curve, which would represent 2 different electrolytes. The second salt might perhaps be carbonate? |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Sun Jan 18, 2009 2:11 pm Sun Jan 18, 2009 2:11 pm | |
| - Quote :
- I don't think anyone has gotten a black powder to form. Most people get a white fluffy precipitant in the first few weeks, then by the end of the 6 weeks digestion, the powder has turned brown.
This seems very interesting to me. I have come across some fluffy precipitates forming inside potassium carbonate solutions after it passed through cycles of absorbing dew and evaporation. Within 3-4 cycles, I could get a white fluffy precip to form, but in an experiment that lasted for months and the carbonate passed through many cycles, the precip was brown. Naturally comes the question in my mind if this precip is similar to the one obtained from dew and salt. Is it possible to show some photos of this precip in order to compare it? 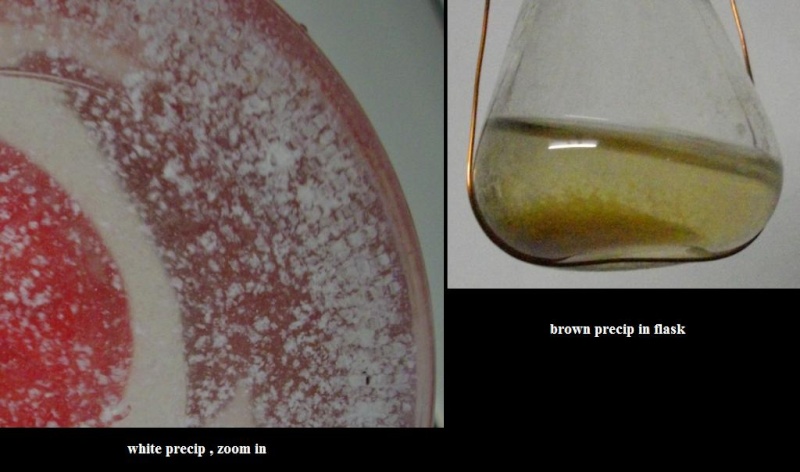 |
|
  | |
NDC
Admin

Number of posts : 599
Age : 43
Location : beyond the veil
Registration date : 2008-12-26
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Sun Jan 18, 2009 4:27 pm Sun Jan 18, 2009 4:27 pm | |
| No. the precipitant from potassium carbonate has absolutely nothing in common with that produced by the dew and salt method. Potassium carbonate eats away at the glass container, and produces silicon dioxide precipitant. The brown stuff comes from the organic material being putrefied and destroyed by the potash.
There is nothing of value in that precipitant. | |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Mon Jan 19, 2009 7:40 am Mon Jan 19, 2009 7:40 am | |
| - Quote :
- Potassium carbonate eats away at the glass container, and produces silicon dioxide precipitant.
I have observed this effect when digesting a concentrated K2CO3 solution. It creates a white salt on the glass which is not fluffy. Upon removing this salt, the glass remains behind eroded and loses its transparency. The precip mentioned in the above post (after washed 4 times with water and dried) reacts with hydrochloric acid, fizzes and finally dissolves in. It doesn't erode the glass. So, maybe it is undissolved K2CO3 or other carbonate. |
|
  | |
Guest
Guest
 |  Subject: Bain Maree (Balneo Mary) Update Subject: Bain Maree (Balneo Mary) Update  Wed Jan 21, 2009 1:17 am Wed Jan 21, 2009 1:17 am | |
| Photo below shows 2 processes in inexpensive 1 litre Schott Duran ‘pyrex’ lab jars. These screw-top jars withstand pressure up to 140°c (284°F). Also shown is dew process in a 1 litre flask with condensing tube and wired-on stopper.  |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Wed Jan 21, 2009 3:05 am Wed Jan 21, 2009 3:05 am | |
| - luce7 wrote:
- Photo below shows 2 processes in inexpensive 1 litre Schott Duran ‘pyrex’ lab jars. These screw-top jars withstand pressure up to 140°c (284°F). Also shown is dew process in a 1 litre flask with condensing tube and wired-on stopper.
cool where did you get those Schott Duran ‘pyrex’ lab jars from? and whats the condensing tube for? |
|
  | |
Guest
Guest
 |  Subject: :-) Subject: :-)  Wed Jan 21, 2009 5:51 am Wed Jan 21, 2009 5:51 am | |
| Great equipment luce7  and thank you for showing us some pics of it. |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Wed Jan 21, 2009 8:46 am Wed Jan 21, 2009 8:46 am | |
| Hi luce7,
The screw-top can resist the pressure but how about the jar ? I have heard -1 to +1,5 bar for that kind of bottles ( http://www.gazettelabo.fr/hybride/nom/s/schott/Flacons_Capuchons.pdf see page#4) Is it enough for our precess ?
[quote="luce7"]Photo below shows 2 processes in inexpensive 1 litre Schott Duran ‘pyrex’ lab jars. These screw-top jars withstand pressure up to 140°c (284°F). Also shown is dew process in a 1 litre flask with condensing tube and wired-on stopper. |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Wed Jan 21, 2009 8:13 pm Wed Jan 21, 2009 8:13 pm | |
| Schott Duran lab jars are widely used in labs all over the world and should be available from any decent lab supplier. I get 1 litre jars here in Australia for US$10 each. Since our process uses 200°F (93°c), there should be no problem with exploding jars or caps.
The 1 litre ‘quickfit’ flask and condensing tube setup is possibly the best way to go (according to Nick) and this is because the condensing tube allows an excellent circulation of the dew at a lower pressure than with jars and bottles. |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Thu Jan 22, 2009 1:13 am Thu Jan 22, 2009 1:13 am | |
| - luce7 wrote:
- Schott Duran lab jars are widely used in labs all over the world and should be available from any decent lab supplier. I get 1 litre jars here in Australia for US$10 each. Since our process uses 200°F (93°c), there should be no problem with exploding jars or caps.
The 1 litre ‘quickfit’ flask and condensing tube setup is possibly the best way to go (according to Nick) and this is because the condensing tube allows an excellent circulation of the dew at a lower pressure than with jars and bottles. Thanks... I am in Australia too, if you bought online can you link me to the site you bought from? I checked the Aunet site and some others and they only sell in packs of 10. |
|
  | |
Guest
Guest
 |  Subject: Lab Jars Subject: Lab Jars  Thu Jan 22, 2009 8:21 am Thu Jan 22, 2009 8:21 am | |
| Hi Furion, I buy direct from a small supplier in Melbourne called LABQUIP but they sell online also at www.labquipsales.com.au or you can phone them on 03 9729 9799.  |
|
  | |
Guest
Guest
 | |
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Mon Feb 02, 2009 11:32 pm Mon Feb 02, 2009 11:32 pm | |
| - NDC wrote:
- Certainly by far, the dew collected from the tips of pine needles is the best. The needles charge the dew so strongly that even drinking the dew it self has an affect.
fortunately there are giant pine trees in my yard. my first idea for the collection of the dew is to spread a tarp and simply shake the tree limbs, however experienced guidance is gratefully appreciated. |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Tue Feb 03, 2009 4:02 pm Tue Feb 03, 2009 4:02 pm | |
| I have been working with essential oils for many years and when I heard of the amazing benifits of Siberian Cedar oil...I had to give it a try. I can honestly atest to the healing benifits of the oil, so getting dew from this source would imprint the dew. Now going to Siberia is a bit far fetched but I also communicate (feel the frequency) of such great entities. For those of you who are interested other cedars are of simular components.
Cedar oil contains a unique combination of biologically active substances which are critical in the health of humans.
Ingredients Include: Vitamins: A, B1, B2, B3(PP), D, E, F; micro- and macro elements (phosphorus, iodine, copper, magnesium, zinc, cobalt, manganese etc.); Polyunsaturated fatty acids; vegetable proteins and fats.
Traditional uses: for prevention of atherosclerosis; optimization of metabolism; normalizing nervous system function; reducing cholesterol; improving functionality of cardiovascular system; for treating chemical injuries, burns and chilblains; for mental tiredness; for enhancing work and mental efficiency; for allergic disorders; for treating tracheitis, laryngitis, acute respiratory diseases; for children to stimulate growth;
Massage with cedar oil helps fatigue and improves peripheral blood circulation; increases lymphatic flow and eliminates venous congestion in limbs; improves skin elasticity; contributes to rejuvenation of skin and helps heal wounds. |
|
  | |
NDC
Admin

Number of posts : 599
Age : 43
Location : beyond the veil
Registration date : 2008-12-26
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Mon Feb 16, 2009 10:13 pm Mon Feb 16, 2009 10:13 pm | |
| The reason you don't see much salt forming on the bottom is probably because you used such a small amount of dew. Leyden used a pound and a half (about 720ml) which is nearly 3 times what you have used. Also you are only suppose to fill the flask 2/3. | |
|
  | |
NDC
Admin

Number of posts : 599
Age : 43
Location : beyond the veil
Registration date : 2008-12-26
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Mon Feb 16, 2009 10:46 pm Mon Feb 16, 2009 10:46 pm | |
| The air provides more room for circulation of the spirit, and this may be what allows the solution to change.
But at any rate, I don't think the precipitant is all that important to the process, and I don't think it actually contains any of the Alkahest. In his letter Actum Leyden had a great deal to explain about the salt on the bottom, and maybe he believed it was the Alkahest itself, but since everyone's salt water seems to produce different types of precipitant, I don't think it actually matters what color it is or how much their is, but I could be wrong.. | |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Thu Feb 19, 2009 8:55 am Thu Feb 19, 2009 8:55 am | |
| Hi. I'm a complete "noob" in this subject and English isn't my native language so forgive me for asking "dumb" questions. I've read the book, but there are some things I just don't understand or which are not described in detail. "Balneo Mary Bath": Do you really have to keep the heater running 24/7 for several weeks? Whats a "Sand bath"? (yes, I can see the photo and read the description above but I still don't understand) Do you have to buy this thing or is it just one out of 1000 possibilities to make a "sand bath"? I'm a student, living in a very small flat in a major city. There's an extremely small balcony (~1.5m²), so collecting dew shouldn be the greatest problem. But I have no idea where I should place a bathtublike "Balneo Mary Bath" construction thats running 24/7. When I collect dew in a bottle, should it be out of a special material? Glass, plastic,..? Can you put the dew-bottle in a fridge to not exceed the 70°F? The cycles described in point 6: How long do they take? 2 weeks each? So it would take around 18 weeks to have a purified mixture + an unmentioned amount of time for point "7", constantly running either the Balneo or the sand bath? Wow, thats going to be a really tough discussion with my girlfriend explaining the sense of having such a gigantic electricity bill..  Doesn't even sound close to be as easy as it's said or maybe I'm just having a great bunch of bad luck.   Thx for your answers and maybe somebody has an idea how I could still make it without throwing my girl out of her flat?  |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Thu Feb 26, 2009 9:29 am Thu Feb 26, 2009 9:29 am | |
| Hello Smoothambiguity!
Are you using your heating mantle for the balneum marie as well? or you take it out of there and use another device?.
Are you using a rubber stopper in that flask ?
thanks ! |
|
  | |
NDC
Admin

Number of posts : 599
Age : 43
Location : beyond the veil
Registration date : 2008-12-26
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Fri Mar 06, 2009 2:20 am Fri Mar 06, 2009 2:20 am | |
| Smooth was deleted from the forum for posting immature sexual comments in refference to the Holy elixir. | |
|
  | |
Guest
Guest
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  Sat Mar 07, 2009 11:18 pm Sat Mar 07, 2009 11:18 pm | |
| |
|
  | |
Guest
Guest
 |  Subject: must the sandbath be dry?? Subject: must the sandbath be dry??  Mon Mar 16, 2009 12:16 pm Mon Mar 16, 2009 12:16 pm | |
| Hello
Im now planing the process. Would it be useful to buy a thermostate, to create a bath. now if i would buy a thermoste, which is able to hold the temperature of 200°F -> 93.3333 °C.
So it would not be a dry bath, but it would be very exactly on temperature. what do you think about this?
How do you solve this Problem?
[img][/img] |
|
  | |
Sponsored content
 |  Subject: Re: Updated: Balneo Mary Bath Subject: Re: Updated: Balneo Mary Bath  | |
| |
|
  | |
| | Updated: Balneo Mary Bath |  |
|
